Abstract
Background. Pyruvate Kinase deficiency (PKD) is a rare congenital hemolytic anemia which affects approximately three people per million individuals worldwide. With no disease-modifying treatments currently available, management tends to focus on supportive symptom control such as blood transfusions. PKD has a profound, wide-ranging impact on quality of life (QoL). Patient advocacy and patient-reported outcomes research has been limited. The PKD Advocacy Advisory Council (AAC), a group of patients, caregivers, patient advocates and physicians, was formed in 2020, by Agios Pharmaceuticals, to improve both timely diagnosis and access to education, support and care for individuals with PKD.
Objective. The AAC initiated a survey to explore communication between patients/ caregivers affected by PKD and their hematologists to inform future best practices and improve outcomes.
Methods. A web-based, quantitative and qualitative survey was conducted amongst adult patients with PKD, and adult caregivers, with respondents from 11 countries including France, Germany, Italy, Spain, the UK and US. Question types included closed-ended, multiple choice, Likert scale and binary choice plus free text. The survey was carried out according to British Healthcare Business Intelligence Association Legal and Ethical Guidelines, as well as guidelines established by the UK's Market Research Society. Participants were recruited via online panels and via AAC member channels, including PKD Facebook groups.
Results: The survey was completed by 200 adult patients with PKD and 75 adult caregivers (n=275). Twenty percent of patients were >50 years of age, 64% were ages 31-50 years and 17% were 18-30 years of age. Half of the patients had been diagnosed for >10 years. Although 82% of patients reported that their hematologist manages PK deficiency "well", the survey revealed gaps in hematologists' understanding. Only 56% of respondents stated their hematologist is able to answer disease management questions and only 44% say their hematologist searches for and finds solutions to optimize disease management. Less than half of respondents, 44%, reported that their hematologists demonstrate a deep knowledge of PKD.
The survey revealed an unmet need regarding emotional and psychosocial support with 25% of respondents reporting feeling neither positive nor negative, or somewhat negative, after hematologist interactions and 29% reporting at least one negative emotion following interactions. After meeting with their hematologist, 21% of respondents report feeling worried, 17% anxious, and 17% depressed.
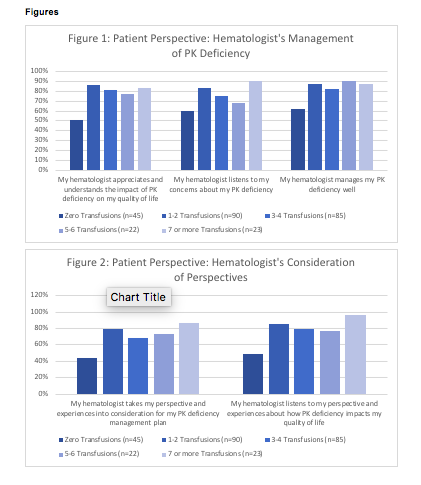
Communication with the hematologist was reported to be negative most often among patients who receive 0 transfusions per year. Within this group, only 62% report that their hematologist manages their condition well compared to 82% overall (p=0.003). Only 51% state that their hematologist understands the impact of PK deficiency on their QoL (compared to 83% amongst respondents receiving one or more transfusions per year, p<0.001). Of those who are not transfused, only 44% state their hematologist takes their perspective and experiences into consideration for their disease management plan (compared to 75% amongst respondents receiving one or more transfusions per year, p<0.001).
Conclusions
PKD is a lifelong chronic disease that significantly impacts QoL of patients and families. The results of this largest ever survey of patients and caregivers with PKD point to a need to adapt clinical approaches to improve outcomes. Hematologists should seek to improve their understanding of the disease and its burden, particularly in non-transfused patients. Care should attend to emotional and psychosocial health aspects. Clinicians should consult with other medical specialists, including hematologists who specialize in PKD, to ensure complications are managed effectively. More research is needed to build on the survey insights, drive further awareness and understanding of the needs of those living with PKD among hematologists, and inform approaches to improve outcomes.
The authors would like to acknowledge and thank members of the AAC that made this work possible.
Grace: Dova: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Agios: Research Funding; Principia: Membership on an entity's Board of Directors or advisory committees. Barcellini: Novartis: Honoraria; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Research Funding; Alexion Pharmaceuticals: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees.